Decomposition of Hydrogen Peroxide Equation
Cus H2O2aq 2 Haq Cu2aq 2 H2Ol In the presence of muratic acide I expect youll also notice bubbles forming on the surface of the copper. S18 but the decrease on the ozone conversion of 750 MnO 2 GR starts after 20 h which.
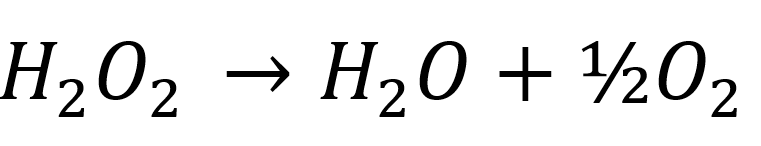
Decomposition Performance Of Hydrogen Peroxide For Use In Bi Propellant Thrusters By Themagicnacho Medium
The decomposition is determined to occur at half-lives of 12 24 and 48 seconds.
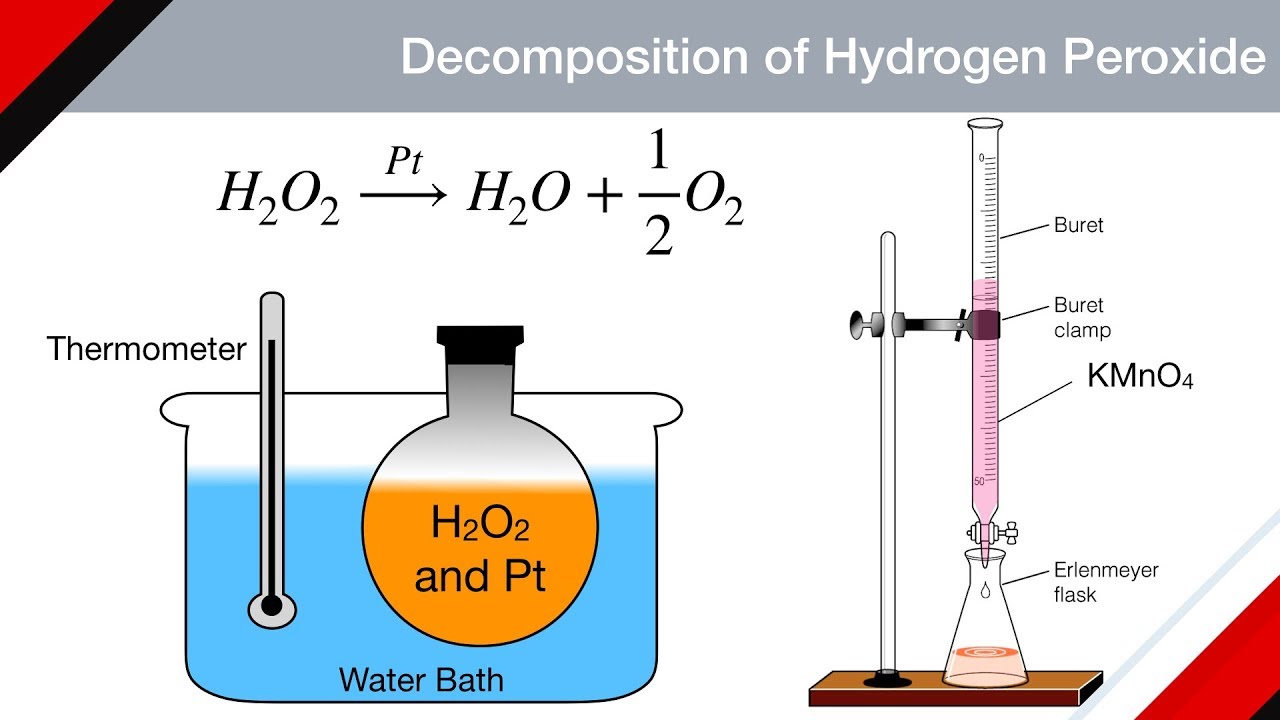
. Collect the hydrogen gas by inverting a water-filled tube or jar over the wire producing the hydrogen gas. 2H 2 O 2aq- 2H 2 O l O 2g. AB A B is a general equation that can be used to represent this.
Other reactions involving decomposition do require the input of. In the presence of light hydrogen peroxide decomposes into water and oxygen. Data for the decomposition of hydrogen peroxide at some set temperature T is provided below.
What is the difference between displacement and double displacement reactions. If the pressure of NO2 is initially 746 torr in a 500L container at 383C a Write the rate law for the reaction b What is the molar concentration of NO2 after 24 seconds c What is the molar concentration of NO2 after 96. If Hydrogen Peroxide levels are too high then consider inserting copper metal.
3 The decomposition of NO2 produces NO gas and oxygen gas. Peresters are the peroxy analog of esters and have general structure RCOOOR. The other bubbles are impure oxygen.
The one with more bubbles is giving off pure hydrogen. These same data will be used for questions 6 7 and 8 2 H2O2 --- 2 H2O O2 t seconds o H2O2 M 0882 0697 0566 0458 0372 0236 0188 0094 60 120 180 240 360 420 600 What is the. This reaction is one of the exceptions to the endothermic nature of decomposition reactions.
How many moles of O₂ are required to form 500 moles of H₂O. 2 H 2 O 2 2 H 2 O O 2. The peak intensity associated with the peroxide species reaches the maximum intensity after 2 h Fig.
The rate law depends only on the concentration of H2O2. An example of a spontaneous without addition of an external energy source decomposition is that of hydrogen peroxide which slowly decomposes into water and oxygen see video at right. The hydrogen bubbles will burn.
Equation for the Reaction Between Baking Soda and Vinegar. Decomposition reactions are the breakdown of chemical species into simpler. Organic peroxides are organic compounds containing the peroxide functional group ROOR.
Copper reacts with hydrogen peroxide to form water and copper ions plus releasing Oxygen. Either from light heat or electricity most of the decomposition reactions require energy. For example in the reaction.
The mole ratio between H₂ and H₂O is 2 mol H₂2 mol H₂O. Hydrogen peroxide H 2 O 2 is a versatile and ecofriendly clean oxidizing agent which has been widely used in chemical synthesis renewable energy and wastewater treatment Therefore the demand for the H 2 O 2 has been continually increasing and the market demand of H 2 O 2 is expected to reach 6000 kilotons in 2024 At present H 2 O 2 is mainly produced. 2H₂g O₂g 2H₂Og The mole ratio between O₂ and H₂O is 1 mol O₂2 mol H₂O.
Once the values of actual yield and theoretical yield are discovered following through with the rest of the equation becomes fairly easy. A Thermal decomposition reaction Thermolysis. The companys chemist puts this information in the percent yield.
The decomposition of hydrogen peroxide into water and oxygen happens in the presence of light. Which is decomposition reaction. The decomposition of hydrogen peroxide by itself is.
Shifting electrochemical oxygen reduction towards 2e pathway to hydrogen peroxide H2O2 instead of the traditional 4e to water becomes increasingly important as a green method for H2O2. 2 HAnother example of this type of reaction is the spontaneous decomposition of hydrogen peroxide into water and oxygen. Write one equation each for decomposition reactions in which energy is supplied in the form of heat light or electricity.
You can test which gas is hydrogen by lighting a match or lighter over the container. Mole ratios are used as conversion factors between products and reactants in stoichiometry calculations. We were able to determine our activation energy by manipulating the Arrhenius equation around to be in the form of y mx b.
In a chemical equation the left-hand side will represent the reactants and the right-hand side will represent the products of the reaction. If the R is hydrogen the compounds are called hydroperoxides which are discussed in that article. The oxygen bubbles will not burn.
A decomposition reaction is a type of chemical reaction in which one reactant yields two or more products. The theoretical yield of the hydrogen peroxide decomposition is 543. After measuring the actual yield during the reaction it is 237.
Hydrogen peroxide H 2 O 2 is a ubiquitous molecule in nature that shapes the redox state of planetary surfacesGiven that H 2 O 2 is a major oxidant isotope effects associated with H 2 O 2 chemistry play a key role in determining triple oxygen isotopic compositions δ 17 O and δ 18 O of secondary aerosols and minerals which are powerful proxies for understanding. The breakdown of hydrogen peroxide to water and oxygen as well as the breakdown of water to hydrogen and oxygen are examples of decomposition reactions. This form is where lnk is y 1T is x.
The OO bond of peroxides easily breaks producing free radicals of the form RO the dot represents an.

Kinetics Of Decomposition Of Hydrogen Peroxide Chemical Kinetics Physical Chem Youtube
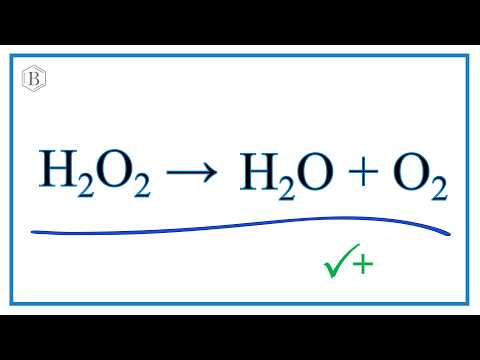
How To Balance H2o2 O2 H2o Decomposition Of Hydrogen Peroxide Youtube

Multimedia A Catalyst And The Rate Of Reaction Chapter 6 Lesson 5 Middle School Chemistry

How To Balance H2o2 O2 H2o Decomposition Of Hydrogen Peroxide Youtube
0 Response to "Decomposition of Hydrogen Peroxide Equation"
Post a Comment